Topical Imiquimod as Adjunct to Conventional Chemotherapy, an Effective Treatment for Cutaneous Invasive Breast Carcinoma Metastasis: A Case Report
- PJO
- Jun 14, 2025
- 11 min read
Updated: Dec 1, 2025
Original Article
Topical Imiquimod as Adjunct to Conventional Chemotherapy, an Effective Treatment for Cutaneous Invasive Breast Carcinoma Metastasis: A Case Report
1 Department of Dermatology, East Avenue Medical Center, Metro Manila
Corresponding author: Joanne Abigail R. Pamin; paminjoanne@gmail.com
ABSTRACT
Background: Breast cancer is the most diagnosed cancer worldwide and has the most significant cancer burden for women. As much as 70% of female breast cancer patients will develop cutaneous metastasis. Despite the myriad of therapeutic options, few have demonstrated the synergistic effect imiquimod 5% cream has on cutaneous metastatic prognosis in addition to chemotherapy.
Case Discussion: Herein is a case of an ER/PR negative and HER2NEU positive patient who presented with cutaneous invasive breast carcinoma metastasis to the right chest wall. The patient developed cutaneous papules and patches despite already having undergone multi-modal treatment. The patient first underwent a modified radical mastectomy. Next, the patient received chemotherapy with fluorouracil, epirubicin hydrochloride, and cyclophosphamide (FEC-D), followed by docetaxel. The patient also received radiation therapy alongside a trastuzumab regimen. After cutaneous lesions developed, the doctor referred the patient to dermatology. There, a histological evaluation revealed findings consistent with cutaneous metastasis. The medical oncologists then added topical 5% imiquimod cream to the ongoing trastuzumab and capecitabine therapy, which the patient tolerated well. In just three months, the patient, demonstrating remarkable resilience, experienced tumor regression, improved quality of life, and even survived beyond the typical prognosis for this condition.
Conclusion: This case highlights the potential of topical 5% imiquimod cream as a minimally invasive and effective local therapy for cutaneous breast carcinoma metastasis.
KEYWORDS: Imiquimod; Capecitabine; breast carcinoma; cutaneous metastasis
INTRODUCTION
Cutaneous metastasis accounts for two percent of skin tumors, with an overall incidence of 5.
3% in cancer patients. In females with breast cancer, cutaneous metastasis develops in up to 70% of cases.(1) Since 2013, cutaneous metastasis has risen by 45% from 0.9%. This trend is expected to continue.(2) Thus, clinicians need a high index of suspicion for recognition, a low threshold for biopsy, and the capability to optimize cutaneous metastasis management.
Metastatic dissemination may be the first sign of underlying malignancy. Notably, when the primary is of the breast, the most common location for metastasis is the anterior chest. It may present as patches, papules, ulcers, nodules, induration of the skin, or even hair loss. Of those cases,0.6% arise from the mastectomy scar. Other concurrent metastases develop in the lungs, liver, and lymph nodes.(3) Thus, a complete body evaluation is prudent. Though rare, cutaneous metastasis usually presents in the later stages of cancer. Its development heralds a poor prognosis as patients tend to die from the malignancy in six months or within the first two years. The treatment objective is palliative, expressly to relieve symptoms, improve quality of life, slow cancer growth, and prolong life. Topical imiquimod 5% cream, a well-documented effective treatment for actinic keratosis and superficial basal cell carcinoma, holds promising potential as a specific treatment for cutaneous metastasis. This case demonstrates the treatment of an ER/PR negative and HER2NEU positive patient with cutaneous invasive breast metastasis using imiquimod adjunctively, sparking further interest in its potential applications.
CASE DESCRIPTION
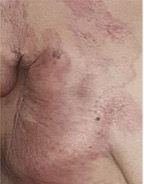 |  |  |  |
S/P Mastectomy, Right Breast, S/P FEC 100, S/PRadiation Therapy, | Imiquimod 5% BID on weekdays, Mupirocin ointment BID x 7 days, NSS compress BID for 20 mins, | Imiquimod 5% BID on weekdays, Mupirocin ointment BID x 7 days, NSS compress BID for 20 mins, | Imiquimod 5% BID on weekdays for 3 months (last application July 25, 2023) |
Ongoing Trastuzumab 600 mg SQ | Ongoing Trastuzumab 600 mg SQ | Capecitabine 500 mg 2 tabs AM, 1 tab PM + Trastuzumab 600 mg SQ | Ongoing Capecitabine 500 mg 2 tabs AM, 1 tab PM + Trastuzumab 600 mg SQ |
FIGURE 1 Clinical progression. (circles = topical treatment area)
The patient is a 63-year-old Filipino female with invasive breast carcinoma of the right breast, stage IIIC. Co-morbidities include hypertension and diabetes. Unfortunately, the patient's mother died before medical oncologists could diagnose her breast mass. She mothered and breastfed one son. Otherwise, the patient's medical history is unremarkable. Two months following diagnosis, the patient underwent a modified radical mastectomy, and all twelve of the dissected lymph nodes were positive. Immunohistochemical stain revealed ER/PR negative HER2NEU strongly positive. The patient received six cycles of FEC-D chemotherapy followed by 34 sessions of radiation therapy.
Doctors started additional immune-targeted therapy of trastuzumab 600mg subcutaneously every 21 days to treat HER2NEU overproduction.
After her fourth trastuzumab session, the patient developed multiple papules and plaques associated with mild pruritus over her right chest. In the interim, the plaques spread to the right shoulder and back. A solitary lymph node over the right clavicle became palpable.
Nine months since cutaneous lesion onset in March 2023, referral to Dermatology service for suspected locoregional recurrence prompted skin punch biopsy. Baseline bloodwork showed anemia, leukopenia, and marginally elevated total bilirubin. Breast and liver ultrasound and chest x-ray were unremarkable. Chest computed tomography (CT) scan detected mildly nodular thickening of the mastectomy bed. The presence of solitary pre-sternal nodules and enlargement of a few axillary and mediastinal lymph nodes at the right paratracheal region was detected.
 |
Histopathologic evaluation revealed nests of pleomorphic atypical cells, consistent with cutaneous metastasis, probably secondary to invasive ductal carcinoma. Subsequent CK7 staining revealed robust cytoplasmic staining in neoplastic cells, while CEA indicated moderate to intense cytoplasmic staining. Adjuvant imiquimod 5% cream was initiated. Initially, this was applied twice daily on weekdays over the papules on the right chest (encircled in Figure 1).
After two weeks of applying topical imiquimod cream, the medical oncologist added oral capecitabine 1500mg daily in three doses to the patient's ongoing trastuzumab therapy. There was marked improvement, as physical examination showed papular regression with erosion and crusting. Imiquimod application was thus expanded beyond papules to all affected areas of the trunk and back. Imiquimod was well tolerated; the patient only reported mild stinging, which went away independently.
After three months of adding imiquimod treatment, the patient's condition resolved. However, there was post-inflammatory hypo-to hyperpigmentation (skin discoloration) where the papules used to be. Plaques had resolved as hyperpigmented patches. Repeat chest CT revealed no sign of tumor recurrence, and abdominal CT was unremarkable. Ideally, histopathologic examination with staining is done to document regression on top of imaging, but priority is placed on affording chemotherapy treatment.
DISCUSSION
Herein, we present a case of ER/PR negative HER2 strongly positive cutaneous metastatic breast cancer treated successfully using topical immunotherapy. Our patient underwent three months of topical imiquimod 5% therapy twice daily on weekdays. As an adjunct to trastuzumab and capecitabine, the regimen was well-tolerated. This case demonstrates the efficacy of topical imiquimod immunotherapy as an adjunct to conventional chemotherapy in the treatment of cutaneous invasive breast cancer metastasis.
Immunotherapy in breast cancer is an actively evolving field. Localized imiquimod, along with 5-fluorouracil and the newer interleukin therapies, are among the options demonstrated to be effective adjuncts to chemotherapy. Imiquimod is of interest due to its efficacy, ease of access, and local availability. It is an immune response modifier with potent anti-tumor activity via toll-like receptors, resulting in apoptosis. Primarily, imiquimod activates the intrinsic pathway of the caspases to create an apoptosome, which destroys malignant cells. Topical application leads to localized increased expression of proapoptotic proteins of the Bcl-2 family.(4) It also directly stimulates Langerhans cells to activate the adaptive immune response, aside from increasing cytokines, IL-12, TNF-alpha INF-gamma, and IL-10, which stimulates anti-tumor T cells and natural killer cells.
Imiquimod emerges as a promising alternative to surgery for treating pre-cancerous and cancerous skin lesions. Studies have documented its effectiveness as monotherapy for squamous and basal cell carcinoma, achieving cure rates between 42% and 100%. Depending on the frequency and the six or 12-week duration of application, 80%-93% of patients were in remission with high tolerability.(5,6) It is commonly combined with five fluorouracil, gentian violet, chemotherapy, and physical therapy like cryotherapy for advanced primary cancers or metastasis. Literature has various examples of efficacy studies on metastasis from primary colon cancer, malignant melanoma, Merkel cell carcinoma, metastatic renal cell carcinoma, and more.(7)
Specifically for breast carcinoma, imiquimod stimulates dendritic cells and macrophages, releasing inflammatory cytokines and enabling apoptosis. Various studies have demonstrated the effectiveness of imiquimod. Application three times a week as an adjunct to chemotherapy resulted in decreased lesion thickness and pain score.(4,8) Nguyen et al. reported using topical imiquimod to successfully shorten the ductal carcinoma in situ (DCIS) duration. In their study, patients with painful, hardened, and ulcerated plaques experienced resolution within four months, suggesting imiquimod's potential as a needed topical treatment for such cases. Regression of the cutaneous metastasis was documented to regress as early as 3 weeks.(8) Drohan et al. demonstrated cutaneous regression in three months with intralesional IL-2 injections biweekly in combination with imiquimod treatment over 32 weeks.(9) Krishnasamy et al. conducted a case series investigating a synergistic response to systemic therapy. The case series showed that combining cryotherapy with imiquimod and 5-fluorouracil improved cutaneous lesions in three women within four months, with sustained improvement lasting up to six months.(10) Our case demonstrated increased efficacy of topical imiquimod added to chemotherapy, resulting in tumor regression compared to untreated areas with no regression, only hyperpigmentation. It was well tolerated, with only localized irritation and erosions and clinical regression of erythema, induration, and papules in the applied area.
This case’s oncologic management also demonstrates optimized management to delay cancer progression and further metastasis. The National Comprehensive Cancer Network (NCCN) Panel recommends post-mastectomy radiation to the chest wall in all surgical breast carcinomas. Radiation therapy after mastectomy and axillary node dissection reduced both recurrence and breast cancer mortality in patients with positive lymph nodes.(11) According to Dr. Coudert and his colleagues, FEC-D in HER2NEU(+) and node-positive patients results in a 15% relative risk reduction of relapse and a 25% relative risk reduction in the relative risk of death at year eight.(12) The patient's aggressive cancer prompted the addition of trastuzumab to her treatment regimen.
Trastuzumab, a drug used to improve survival since the 1990s(13), has shown positive outcomes in similar cases involving HER2NEU overexpression. Studies reveal that patients treated with trastuzumab experience delayed disease progression, longer response durations, higher overall response rates, lower death rates at one year, and a reduced risk of death compared to those receiving standard chemotherapy alone.(14,21)
The confirmation of metastasis coincided with the initiation of trastuzumab therapy combined with capecitabine. Capecitabine is an oral prodrug of fluorouracil, which is FDA-approved for the treatment of metastatic breast cancer. It is effective after anthracycline- or taxane-based regimens, as in our patients. Musada and colleagues have demonstrated that those treated with capecitabine compared to anthracycline and taxanes achieved longer disease-free survival times. In the capecitabine group, a higher percentage were alive without recurrence or secondary cancer at five years compared to the control (74.1% vs. 67.6%). Similarly, the overall survival rate was 78.8% versus 70.3%.(15) Overall response rate for continuous capecitabine was 20% and 18% for a cyclophosphamide, methotrexate, and 5-fluorouracil regimen. Treatment duration was more likely to continue beyond six months in monotherapy capecitabine-treated patients versus combination therapy, but the former has a shorter median time to progression (TTP) of >4 months or even as early as 3.1 months and lower overall response rates.(16) As for the combination of trastuzumab and capecitabine, there is an expected 23% tumor shrinkage and 27% increase in the two-year survival rate for HER2NEU-positive patients. This combination effectively increases survival in patients who have already received multi-modal treatment.(13) The combination of trastuzumab and capecitabine had a median TTP of 8 months but no significant difference in overall survival for combination treatment with a standard taxane-containing regimen.(17) This is similar to the duration of trastuzumab therapy, where the times to progression were 7.25 and 5.25 months for the first and second lines of trastuzumab therapy.(18)
Generally speaking, the prognosis of cutaneous metastasis is poor. Mortality is >70% in the first year after diagnosis, and as much as 50% of patients expire within the first six months, with an average survival time of 7.5 months. Treating the primary tumor is essential for any chance of curing cutaneous metastasis.(19) The response to combination chemotherapy varies significantly between patients. Therefore, standardized criteria for evaluating response in cutaneous metastasis and treatment algorithms specifically designed to optimize skin-directed therapies are urgently needed.
As seen in our patient, for the goal of prolonging patient survival and a disease-free state, topical therapy to conventional systemic chemotherapy has been demonstrated to improve the disease prognosis rapidly. Combination treatment is generally well tolerated, and imiquimod 5% cream treatment improved quality of life. This case demonstrates that adjunct imiquimod 5% cream applied twice daily on weekdays with systemic chemotherapy can achieve disease regression in three months, more rapidly than previously documented.
CONCLUSION
Imiquimod therapy proves to be an effective complementary treatment for cutaneous metastasis from invasive breast carcinoma. In this case, combination treatment resulted in tumor regression, improved quality of life, and even survival beyond the typical prognosis for this condition. This finding highlights imiquimod cream as a practical option for minimally invasive and effective local therapy in such cases.
However, due to the observation of tumor recurrence six months onwards (TTP noted), long-term follow-up every two years (bi-annually) is warranted. Further research is also needed to investigate the effectiveness of imiquimod as a standalone treatment (monotherapy) and its potential application in treating cutaneous metastases originating from other primary tumors.
DATA AVAILABILITY STATEMENTS
All data underlying the results are available as part of the article, and no additional source data are required.
ETHICS STATEMENT
Written informed consent was obtained from the patient for their anonymized information to be published in this article.
AUTHORS CONTRIBUTION
JAP, writing – original draft, review and editing, resources; Elizabeth P. Prieto, writing – review and editing
FUNDING
This research received no external funding.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to commercial or financial relationships.
PUBLISHER’S NOTE
This article reflects the views and findings of the authors alone and does not necessarily represent the official position of the author's affiliated organizations, the publisher, editors, or reviewers. We encourage readers to remember that the content presented here does not constitute endorsement or approval by any of the entities above.
Similarly, any products or services mentioned within this article are for informational purposes only. The publisher recommends that readers conduct independent evaluations before making any decisions, as the publisher does not guarantee or endorse any products or services mentioned.
DECLARATION OF USE OF GENERATIVE ARTIFICIAL INTELLIGENCE
No generative AI technologies were used in the writing of this manuscript.
REFERENCES
Miguel TS, Costa DA da, Almeida APM de, Pino LC de M, Goldemberg DC, Miguel BS, et al. Erysipelatoid Carcinoma. Rev Assoc Med Bras. 2018;64(6):492–7.
Wong CB, Kalb R, Zeitouni N, Helm M, Helm T. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5(9):499.
Dumlao JKG, Cubillan ELA, Villena JPDS. Clinical and histopathologic profile of patients with cutaneous metastasis in a tertiary hospital in the Philippines. Dermatopathology (Basel). 2022;9(4):392–407.
Bubna A. Imiquimod - Its role in the treatment of cutaneous malignancies. Indian J Pharmacol. 2015;47(4):354.
Murawa D, Połom K, Murawa P. Imiquimod as an alternative to surgery in treating skin cancers – experience with the first group of patients. Contemp Oncol (Pozn). 2011;1(1):27– 30.
Scarfì F, Patrizi A, Veronesi G, Lambertini M, Tartari F, Mussi M, et al. The role of topical imiquimod in melanoma cutaneous metastases: A critical review of the literature. Dermatol Ther. 2020;33(6).
Asakura M, Miura H. Imiquimod 5% cream for treating nasal lesion of metastatic renal cell carcinoma: Imiquimod for metastatic carcinoma. Dermatol Ther. 2011;24(3):375–7.
Nguyen AL, Chong EG, Lee J, Mirshahidi S, Mirshahidi H. A case report of imiquimod topical therapy as a treatment for cutaneous metastasis of breast cancer. Rare Tumors. 2021;13:203636132097574.
Drohan A, Vidovic D, Barnes PJ, Giacomantonio CA, Helyer LK. Cutaneous breast cancer metastasis is effectively treated with intralesional interleukin-2 and imiquimod: A case report and brief literature review. Front Oncol. 2022;12.
Krishnasamy SR, Almazan TH, Suero-Abreu GA, Jung JY. Successful treatment of cutaneous metastatic breast cancer with topical treatments that potentially synergize with systemic therapy: A case series. JAAD Case Rep. 2018;4(7):711–5.
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast Cancer, version 3.2022, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722.
Coudert B, Asselain B, Campone M, Spielmann M, Machiels J-P, Pénault-Llorca F, et al. Extended benefit from sequential administration of docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide regimen for node-positive breast cancer: The 8-year follow- up results of the UNICANCER-PACS01 trial. Oncologist. 2012;17(7):900–9.
New treatments emerge for metastatic HER2+ breast cancer [Internet]. Cancer.gov. 2020 [cited 2024 Jun 14]. Available from: https://www.cancer.gov/news-events/cancer-currents- blog/2020/tucatinib-trastuzumab-deruxtecan-her2-positive-metastatic-breast-cancer
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
O'Shaughnessy JA, Kaufmann M, Siedentopf F, Dalivoust P, Debled M, Robert NJ, et al. Capecitabine monotherapy: Review of studies in first-line HER-2-negative metastatic breast cancer. Oncologist. 2012;17(4):476–84.
Saini K, Chee P. Treatment of locally advanced cutaneous Merkel cell carcinoma with topical imiquimod. JAAD Case Rep. 2021;13:121–3.Cancello G, Montagna E, D'Agostino D, Giuliano M, Giordano A, Di Lorenzo G, et al. Continuing trastuzumab beyond disease progression: outcomes analysis in patients with metastatic breast cancer. Breast Cancer Res. 2008;10(4).
Spratt DE, Gordon Spratt EA, Wu S, DeRosa A, Lee NY, Lacouture ME, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: A meta-analysis. J Clin Oncol. 2014;32(28):3144–55.
Bartsch R, Wenzel C, Altorjai G, Pluschnig U, Rudas M, Mader RM, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25(25):3853–8.
Received: 2023 Nov 15 Received in revised form: 2024 Mar 15 Accepted: 2024 Jun 11
CITATION
Pamin JA, Prieto EP. Topical imiquimod as adjunct to conventional chemotherapy, an effective treatment for cutaneous invasive breast carcinoma metastasis: a case report. Philipp J Oncol [Internet]. 2025 [cited 2025 Jun 15];1(1):e003. Available from:[https://www.philsoconc.org/post/topical-imiquimod-as-adjunct-to-conventional-chemotherapy-an-effective-treatment-for-cutaneous-inva]

Comments