Hypo-fractionated Radiotherapy versus Conventional Radiotherapy in Post-Mastectomy Breast Cancer Patients: A Single Institution Trohoc Study
- PJO
- Jun 21, 2025
- 21 min read
Updated: Dec 1, 2025
Hypo-fractionated Radiotherapy versus Conventional Radiotherapy in Post-Mastectomy Breast Cancer Patients: A Single Institution Trohoc Study
Thomas Jason Chaking Ngelangel (1), Richmarie Grace Uy (1), Stephen Lowell Ciocon 1, Charles Cedy Lo (1), Marc Vincent Barcelona (1), Jaemelyn Marie Fernandez-Ramos (1), Miriam Joy Calaguas (1)
1 Department of Radiotherapy, Jose R. Reyes Memorial Medical Center, Rizal Avenue, Sta. Cruz, Manila
Corresponding author: Thomas Jason Chaking Ngelangel; tj_ngel@yahoo.com
ABSTRACT
Objective: To evaluate the effectiveness and safety of hypo-fractionated radiotherapy (HFRT) versus conventional radiotherapy (CFRT) post-mastectomy among Filipino women with <Stage III breast cancer.
Methods: This is a cohort study on histopathological confirmed locally advanced breast cancer patients who underwent modified radical mastectomy and had negative surgical margins and <8 positive AXLN. Out of 92 patients from the following cohorts: 1) year 2014-2015, with 10 HF- PMRT patients; 2) year 2019, with 12 HF-PMRT patients and 15 CF-PMRT; 3) 2022 Jan-Mar, with 24 HF-PMRT and 31 CF-PMRT - 87 were eligible.
Patients received either HF-PMRT of 43.2 Gy in 16 fractions (2.7 Gy/fraction) to the chest wall and regional nodes or CF-PMRT with a dose of 50-50.4 Gy in 1.8-2.0 Gy/fraction to the chest wall and regional nodes with scar boost added based on high-risk factors.
Primary endpoints were overall survival, disease-free survival, locoregional recurrence, and distant metastasis control rates. Secondary endpoints included acute and late toxicities. Researchers performed an intent-to-treat analysis.
Results: HF-PMRT and CF-PMRT groups were comparable according to age, co-morbidities, clinical symptoms, and performance status; there were more menopausal women and Stage II cases in the HF-PMRT cohort. Median follow-up in months was 23.5 (range=10-98) for the HF-PMRT cohort versus 23 (range=10-39) in the CF-PMRT. All (100%) CF-PMRT patients experienced a significantly higher interruption in their radiotherapy sessions than those in the HF-PMRT cohort (68%), with a median of 8 versus three interruption days, respectively (p<0.003). The two-year overall survival rate was 86.3% for the HF-PMRT cohort and 100% for the CF-PMRT cohort, p = 0.403. There were three mortalities under the HF-PMRT arm (13.6%) and none in the CF-PMRT; all three mortalities were from the 2014-2015 cohort (1 died of brain metastasis after 26.9 months, another died of unknown cause after 47.5 months, and one died due to cardiopulmonary arrest after 17.9 months follow-up). Four-year disease-free survival rates were 68.18% in the HF-PMRT arm and 73.33% in the CF-PMRT arm, p = 0.126; 4-year distant metastasis control rates were 81.8% and 73.3%, respectively, p = 0.126. There was no reported locoregional recurrence. Acute and late toxicities were similar, mainly affecting the skin.
Conclusion: Hypo-fractionated radiotherapy (HFRT) post-mastectomy shows comparable effectiveness and safety to conventional radiotherapy (CFRT) among Filipino women with <Stage III breast cancer, with fewer treatment interruptions. Despite a slightly lower two-year overall survival rate in the HFRT cohort, the disease-free survival and distant metastasis control rates were similar between the two groups, and toxicity profiles were comparable.
Keywords: Breast cancer, post-mastectomy, hypo-fractionated radiotherapy, adjuvant treatment
INTRODUCTION
The standard of care for locally advanced breast cancer patients who have undergone mastectomy is adjuvant radiotherapy to the chest wall with or without regional nodal irradiation (depending on nodal status and risk factors).
The Jose R. Reyes Memorial Medical Center (JRRMMC) Department of Radiotherapy serves at least 150 new patients annually and has a daily load of more than 30 patients. Currently, the standard for radiotherapy of breast cancer is conventional fractionated radiation therapy (CFRT), which delivers radiation in daily doses or fractions of 1.8-2.0 Gy units of absorbed radiation dose. The pivotal randomized trials, the National Comprehensive Cancer Network guidelines, and multiple international and national guidelines still advocate for CFRT to a total dose of 45-50.4 Gy with or without radiation boost to the scar. The total dose for institutions that perform scar boost Is 60-66 Gy; thus, treatment for post-mastectomy cancer patients would last 25-30 weekdays in 5-6 weeks (1).
The duration of treatment for these patients has raised concerns about scheduling and logistics. Due to the large number of patients receiving and seeking treatment and the limitation in the number of patients JRRMMC can treat with the linear accelerator machine, there are instances where patients must wait 4-6 weeks before receiving treatment. The 5-6 weeks treatment period makes it hard logistically for the patients, as many reside very far from the hospital, with difficulty in their daily commute or in finding accommodations within Metro Manila.
The use of post-mastectomy radiation therapy for breast cancer patients to improve locoregional control and overall survival has been well-established in the landmark trials of Danish 82B and 82C by Overgaard et al.. (2-3) and the British Columbia randomized trial by Ragaz et al. (4). It has become routine in treatment facilities that have the capability.
A recent trend towards hypo-fractionated dosing to, initially, whole breast radiation therapy and, to post-mastectomy patients to exploit the relatively low α/β ratio and to allow for shorter treatment times has shown non-inferiority in terms of survival, local control, and toxicity.(5-7) Radiobiological experiments have shown the use of hypo-fractionated doses of radiation therapy (HFRT) to increase adverse effects on late-responding tissues, and this has always been a concern in its application, such that the use of conventionally fractionated doses of radiation therapy is still the most used. (8)
Several randomized trials, (5-7) however, have shown hypo-fractionated doses of radiation for breast cancer patients to be of favorable efficacy and toxicity. This is particularly advantageous in low-to-middle-income countries such as the Philippines, where treatment costs and logistics concerns are prevalent.
Recently, the COVID-19 pandemic has compelled professional societies for radiation oncology, such as the American Society for Radiation Oncology (ASTRO) and the Philippine Radiation Oncology Society (PROS), to issue guidelines and statements regarding the use of hypo- fractionated treatment regimens for various cancer sites (including breast) to decrease the number of patient visits to radiotherapy facilities, to mitigate possible risk of virus exposure and spread, especially among the vulnerable cancer population. (10)
METHODS
This JRRMMC Philippine observational cohort study compared the effectiveness and safety of hypo-fractionated post-mastectomy radiation therapy versus conventionally fractionated post- mastectomy radiation therapy among <Stage III breast cancer patients.
Study Subjects
Study subjects were selected based on specific inclusion and exclusion criteria. Eligible patients had undergone mastectomy for histopathologically confirmed breast cancer with negative margin resection. They must have had lymph node dissection (including sentinel lymph node biopsy) with fewer than eight positive lymph nodes confirmed histopathologically. Patients who consented to participate in the study were included. However, individuals were excluded if they had internal mammary lymph node metastasis, distant metastasis, any active collagen disease, or active double primary cancer (except for carcinoma in situ and bilateral breast cancer). Additional exclusion criteria included concurrent chemoradiotherapy, previous chest irradiation, pregnancy, and potential pregnancy.
The study included two cohorts: patients from 2014-2015 who received hypofractionated radiotherapy (HFRT) post-mastectomy, and patients from 2019 and January-December 2022 who received either conventional fractionated radiotherapy (CFRT) or HFRT post-mastectomy. Researchers thoroughly explained the possible benefits and side effects of both CFRT and HFRT to the patients. Patients were given the choice between CFRT and HFRT, and their consent was obtained accordingly.
Ethical Considerations
The JRRMMC Ethics Committee approved this study. Patients were asked for their consent, and patient confidentiality was upheld.
Treatment Protocol
The radiation treatment protocol delivers a total dose of 43.2 Gy to patients with negative surgical margins, administered in 16 fractions over 22 days to the chest wall and supraclavicular region. The internal mammary lymph nodes are not included in the radiation field. The radiation sources used are 4-6 MV X-rays or Cobalt-60 X-rays. A tangential irradiation method aligning posterior margins is employed. Treatment planning aims to achieve target dose homogeneity within ±7% of the Planning Target Volume (PTV), adhering to this principle as closely as possible. The radiation field primarily focuses on the chest wall, following established guidelines. Importantly, for all patients, the thickness of the lung field within the radiation field must not exceed 3 cm, ensuring minimal lung tissue exposure.
A scar boost was administered to patients with high-risk factors in the conventionally fractionated arm for dose and fractionation. These factors included age under 50, positive axillary nodes, lympho-vascular invasion, or close margins. However, patients in the hypo-fractionated arm did not receive any scar boost. Regarding the irradiation position, patients were immobilized using a breast board in a supine position with the affected side's upper limb raised. The radiation sources employed were 6 MV X-rays and Cobalt-60 X-rays. The irradiation methods varied by target area: a tangential irradiation method aligning posterior margins were used for the chest wall and tumor bed boost, while the supraclavicular field was treated with a single anteroposterior (AP) field. Treatment planning aimed to achieve target dose homogeneity within ±7% of the Planning Target Volume (PTV), adhering to this principle as closely as possible.
Borders of radiation field for the chest wall |
Inner margin | Midline of sternum |
|---|---|
Outer margin | Middle axillary line (1.5-2 cm outside palpable mammary glands) |
Upper margin | Between the upper edge of the acromial extremity of the clavicle and the lower edge of the extremities sternalis claviculae First costal interspace |
Lower margin | 1-2 cm from the lower edge of contralateral breast borders for the radiation field of the supraclavicular area |
Inner margin | The midline extends from the first costal interspace to the thyroid-cricoid groove, and the medial to the sternocleidomastoid muscle includes the lower lymph nodes of the cervical chain. |
Outer margin | From the acromioclavicular joint, bisecting the humeral head, to exclude as much of the shoulder as possible. |
Upper margin | Extend laterally across the neck and trapezius to the acromial process, ensuring the entire supraclavicular fossa is included visually. |
Lower margin | First, costal interspace, abutting the tangential breast field |
Radiotherapy Regimen Given
Conventional Radiotherapy | Hypo-fractionated Radiotherapy |
Total Dose | 50 Gy | 43.2 Gy |
Dose per fraction | 2 Gy | 2.7 Gy |
Number of fractions | 25 fractions | 16 fractions |
Tumor bed boost | 10 Gy (2 Gy per fraction for five fractions) | None |
Overall treatment days | 33 days;40 days (if with boost) | 22 days |
Combination Therapy
Concurrent chemotherapy was not allowed during radiation therapy. However, concurrent endocrine therapy was allowed. Adjuvant or neoadjuvant systemic therapy followed NCCN guidelines at the discretion of the medical oncologist. (1)
Cancellation Criteria for the Protocol
A patient may be removed from the study for several reasons: if they are found to be ineligible based on inclusion or exclusion criteria after initial registration; if concurrent chemotherapy is administered, which could interfere with the study's treatment protocol; if the trial is discontinued due to an adverse event that poses unacceptable risks to participants; if a patient withdraws their consent for participation, exercising their right to leave the study at any time; or if a physician determines that treatment needs to be discontinued for medical reasons, prioritizing the patient's safety and well-being over the study's objectives.
Data collection
Patient data were collected using case report forms (patient demographic profile and eligibility, disease and treatment profile, radiotherapy and compliance to treatment, other cancer therapy, adverse events, and follow-up after one-month post-RT, and every three months after that looking for radiation toxicities, disease progression, and survival), reviewing patient medical records.
Assessment of Effectiveness
The assessment of effectiveness in this study encompasses several key aspects. Locoregional recurrence is any mass observed at the primary site or regional lymph nodes following complete breast treatment, detected through clinical examination or imaging. Evaluation occurs every three months in the first year, biannually from years two to five, and annually thereafter. The evaluation methods include clinical breast examinations, mammography/ultrasound, and pathology assessments when necessary. Disease progression is identified by distant metastasis or locoregional recurrence, documented through symptoms, physical examinations, and imaging.
Survival analysis focuses on two primary metrics: overall survival and disease-free survival. Overall survival is measured from the start of radiotherapy to death from any cause, while disease-free survival spans from the initiation of radiotherapy to disease progression. This interim analysis requires a minimum of one year of survival data to provide meaningful insights into the treatment's effectiveness and patient outcomes.
Assessment of Toxicity
Acute toxicities for each patient were serially monitored during and after treatment (within 90 days from the beginning of treatment). Evaluation of acute toxicities was done once a week during treatment and one month and three months after the end of treatment. A chest x-ray was performed as needed. Hematological toxicities were assessed according to the NCI/CTC version 4.024. The researchers assessed non-hematological toxicities according to the RTOG acute radiation morbidity scoring system25.
Late toxicities for each patient were periodically monitored after treatment (>91 days after treatment). Regions observed were the skin, subcutaneous tissue, lungs, and heart. Late toxicities were assessed every three months for the first year, every six months for the 2nd year after treatment, and at 3rd-5th year once a year. Data assessors use the RTOG/EORTC late radiation morbidity scoring scheme, the Common Terminology Criteria for Adverse Events v4.0 (CTCAE)24, and the LENT-SOMA scale.26-29 For a left-sided breast cancer patient, two- dimensional echocardiography was requested, whereas a 12-lead ECG was requested for a patient with right-sided breast cancer. A chest x-ray was done accordingly.
Statistical Analysis: Sample Size Calculation
The sample size calculator from the Cancer Research and Biostatistics (CRAB) website of the
Southwest Oncology Group (SWOG) Statistics and Data Management Center was used,30 assuming a computation for a one-arm cohort study.
To compute the necessary sample size in a one-arm non-parametric survival study for Kaplan- Meier survival curve analysis, a 3-year accrual time with a four-year follow-up time was used. The alpha was set at 0.05. The null survival probability was set at 0.65, which was the 5-year overall survival rate of post-mastectomy radiation therapy patients from the Danish 82B and 82C trials by Overgaard et al. (1990)2-3. Alternative survival probability was set at 0.832 based on the 83.2% 5- year overall survival rate of the Sun et al. (2017)15, β or power of the study was set at 0.80, and the computed sample size was 39 with an approximate lower critical value of 0.51 and an approximate upper critical value of 0.82; expected attrition rate was 15%. Attrition rate was compensated using the formula: sample size x [1/(1-attrition in decimal)]. A final sample size of 46 was, thus, computed; this sample size was used for both HFRT and CFRT cohorts (46 each).
Data Analysis (Preliminary)
Data was encoded and analyzed using Microsoft Excel and STATA 15.0 statistical software. All valid data were included in the analysis. The null hypothesis was rejected at the 0.05 α-level of significance.
Descriptive statistics was used to summarize the general and clinical characteristics of the patients: frequency and proportion for categorical variables (nominal/ordinal), mean and standard deviation for normally distributed interval/ratio variables such as age, and median and range for non-normally distributed interval/ratio variables such as time from the start of therapy to mortality.
The mean, frequency, and median differences between groups were determined using the independent sample T-test, Fisher's exact/chi-square test, and Mann-Whitney U test, respectively. The Kaplan-Meier survival estimates method was used to construct curves for overall and disease-free survival, locoregional recurrence, and distant metastasis control. Outcome parameters were estimated up to 4 years.
RESULTS
A total of 87 women who underwent mastectomy for breast cancer, with an average age of 49.90 ± 11.57 years, were included in the study; 46 underwent HFRT and 41 CFRT. The HFRT group was more of the menopausal group (78% vs 44%) [p = 0.0017] (Table 1). The two groups were comparable according to age, co-morbidities, performance status, clinical symptoms (Table 1), molecular subtype, laterality, and tumor site (Table 2), adjuvant drug therapy (Table 3).
Three (3) patients were lost to follow-up: two (2) in the HFRT cohort and one (1) in the CFRT cohort.
The most common histopathologic type was ductal carcinoma (98%) (Table 2). Of these, 12 patients (13.79%) were diagnosed with both invasive ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS). Of 72 women with purely IDC, 41 (89.13%) and 31 (75.61%) received HFRT and CFRT, respectively. Of 12 with in situ and invasive ductal carcinoma, 4 (8.70%) had HFRT and 8 (19.51%) CFRT. Those with HFRT had more invasive cancer than those with CFRT (p=0.013). However, both cohorts were comparable in the presence of high-risk factors (Table 2).
Over half of the patients were luminal-HER2neu negative (57.47%). Fourteen patients were HER2neu positive, and five were triple negative. All patients had unilateral breast cancer, typically in the upper outer quadrant (65.52%). The patients who received HFRT had more Stage IIA and IIB patients than those in the CFRT group, who mainly were Stage IIIA and Stage IIIB (p = 0.04).
All patients (100%) who underwent CFRT experienced an interruption in their radiotherapy sessions, significantly higher than in the HFRT group (71.74%). The duration of interruption was also longer in the CFRT group (median eight vs three days) [p < 0.003]. Endocrine therapy, adjuvant therapy, and targeted therapy were comparable between the two groups.
None of the patients in each cohort experienced any locoregional recurrence. Six patients in the CFRT cohort had distant metastases, whereas four in the HFRT cohort had distant metastases (Table 4).
The distant metastasis control rate was 91.30% for the HFRT arm and 85.37% for the CFRT arm, p>0.05 (Figure 2). Three patients with distant metastases in the HFRT arm were from the 2014- 2015 HFRT cohort.
Three out of the 87 patients died, all from the 2014-2015 HFRT cohort (Table 4). Causes of death include one (1) patient with progression of brain metastases at 26.9 months, one (1) died of unknown causes after 47.5 months, and one (1) died of cardiopulmonary arrest after 17.9 months of follow-up. The overall survival rate was 93.48% for the 2014-2015 & 2019 & 2022 Jan-Mar HFRT cohorts and 100% in the 2019 & 2022 Jan-Mar CFRT cohorts, p = 0.403 (Figure 3).
Disease-free survival rate was 84.78% in the HFRT arm and 85.37% in the CFRT arm, p = 0.126 (Figure 4).
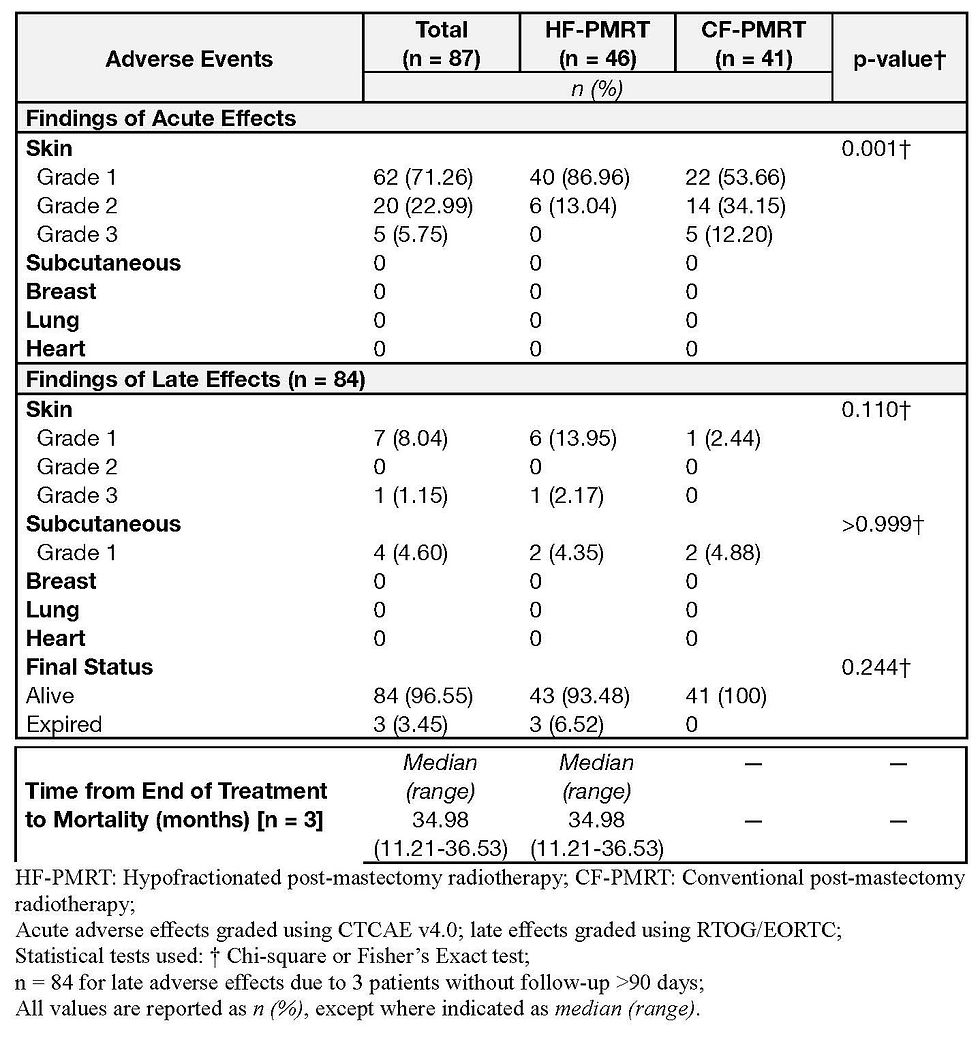
Acute and late toxicities were comparable between the two arms. Grade 1 acute skin toxicities were reported in 95.65% of the HFRT group and 85.37% of the CFRT group. Two patients (4.35%) had Grade 2 skin toxicity in the HF-PMRT group and five (12.20%) in the CFRT group, p = 0.703 (Table 5).
There were no adverse effects on the breast, lung, or heart. There were no significant differences in late toxicities between the two arms (Table 5). The HFRT cohort had six patients with RTOG Grade 1 late skin toxicities, and one patient had Grade 3 late skin toxicity; the CFRT cohort had 1 case of Grade 1 late skin toxicity, p = 0.196. Grade 1 late subcutaneous toxicities were in the HFRT arm (4.35%) and the CFRT arm (4.88%), p = 0.999. Late adverse events were reported in eight patients with skin atrophy, four patients with subcutaneous induration, and one patient with Grade 1 toxicity on the breast. The numerically higher number of Grade 1 late skin toxicities were from the 2014-2015 HFRT cohort, with a more extended follow-up period than the 2019 and 20-22 cohorts from which the CFRT patients came. A five-year follow-up of the cohorts is still ongoing.
DISCUSSION
This JRRMMC Philippine observational cohort study interim analysis (at one year or more follow- up) shows that all the effectiveness endpoints (survival, locoregional recurrence, and distant metastasis control) and safety endpoints (acute and late toxicities) were comparable between the HF-PMRT and CF-PMRT. HF-PMRT promises to be a favorable treatment strategy for breast cancer.
The Chinese HFRT post-mastectomy randomized trial (15) provided the most information on this. It compared a dosing regimen of 43.5 Gy in 15 fractions over three weeks. The 5-year cumulative incidence of locoregional recurrence was 8.3% for the HFRT arm and 8.1% for the CFRT arm, well within the pre-specified level of non-inferiority (p <0.0001). There was no significant difference in the 5-year overall survival (84% vs 86%) and 5-year disease-free survival (74% vs 70%).
The FAST Forward Trial(23) compared three arms: (1) 26 Gy in 5 fractions, (2) 27 Gy in 5 fractions, and (3) 40 Gy in 15 fractions. It noted that the 5-year incidence of ipsilateral breast tumor recurrence was within the pre-specified level of non-inferiority for both experimental arms. However, most of the patients in the study underwent whole breast irradiation rather than post- mastectomy RT (PMRT), making the Beijing HF-PMRT trial(15) the more relevant support literature of this Philippine JRRMMC study.
The two cohorts in this JRRMMC Philippine study were comparable in acute and late toxicities. None of the patients experienced Grade 4 toxicities or higher. The numerical difference in late skin toxicities between cohorts may be due to the longer follow-up of the 2014-2015 HFRT cohort.
The Chinese randomized HFRT postmastectomy trial15 noted a statistically significantly higher rate of Grade 3 acute toxicities in the CFRT arm.
Most data on hypofractionation are for whole breast irradiation, and several studies have shown equivalent or better acute and late toxicity profiles with hypofractionation. (31)
Detecting and grading toxicity is highly important in trials that concern altered fractionation, as the theoretical disadvantage of hypofractionation is an increased rate of late toxicities (5-6,8). Based on this interim JRRMMC Philippine study, that of the Chinese RCT15, and the pivotal trials (5-7,22) of the hypo-fractionated WBI era, this has not been the case so long as the radiation therapy is delivered properly.
There is thus a possible increase in the adoption rate of HFRT postmastectomy, especially in situations like the COVID-19 pandemic, the distance of RT facilities, and productivity loss during long treatment periods.
HFRT post-mastectomy, which has a lower overall treatment time, may reduce treatment interruptions due to external factors or factors out of the control of the treatment facility. Unintended treatment interruptions can prolong overall treatment time, which has been known to lead to inferior clinical outcomes. In a 2017 retrospective study, Rudat et al. compared adjuvant treatment for breast cancer between hyperfractionated radiotherapy (HFRT) and conventional fractionation radiotherapy (CFRT). They had shown that the HFRT arm resulted in better patient compliance, with the fractionation regimen being the only independent significant prognostic factor for compliance. In this JRRMMC Philippine study, treatment interruptions in the CFRT arm were significantly higher.
Breast cancer is still the cancer with the highest incidence globally and in the Philippines, and most Filipino breast cancer patients still present with locally advanced breast cancer and most likely will undergo modified radical mastectomy. This JRRMMC Philippine study's findings add to the growing body of research investigating the similar clinical efficacy of HFRT and CFRT following mastectomy. The study's findings suggest that HFRT following mastectomy could be adopted as a future standard treatment. This adoption of HFRT would have significant implications for patients, their families, and entire healthcare systems. The shorter overall treatment time can decrease the indirect costs shouldered by patients through their daily commutes to the radiotherapy center, especially those from remote provinces. Healthcare systems may be able to experience a reduction in expenses due to the fewer days of treatment needed to treat breast cancer patients, which is the cancer type with the highest number of cases in Philippine radiotherapy centers.
As the study was non-randomized, the comparability between the intervention and control groups was limited. Selection and time period bias might affect the generalizability of results, as might the small sample size. The study is limited to a single government institution.
CONCLUSION
This study comparing hypofractionated radiotherapy (HFRT) with conventional fractionated radiotherapy (CFRT) in Filipino women with stage III or lower breast cancer who underwent mastectomy has yielded promising results. The effectiveness of HFRT appears comparable to CFRT, with no significant differences observed in locoregional recurrence, disease-free survival, or overall survival rates between the two groups. Notably, HFRT demonstrated advantages in terms of treatment adherence, with significantly fewer interruptions and shorter durations of interruptions compared to CFRT.
In terms of safety, both HFRT and CFRT showed similar profiles of acute and late toxicities, with most adverse events being mild (Grade 1) and manageable. The slightly higher number of late skin toxicities observed in the HFRT group may be attributed to the longer follow-up period for this cohort. These findings suggest that HFRT could be a viable alternative to CFRT in this patient population, potentially offering benefits such as improved treatment compliance and reduced burden on healthcare resources without compromising oncological outcomes or patient safety. However, longer-term follow-up data, particularly for the more recent cohorts, will be crucial to confirm these initial findings and to assess any potential differences in late effects between the two treatment approaches.
DATA AVAILABILITY STATEMENTS
Not publicly available
ETHICS STATEMENT
The Ethics Review Board/Committee of Jose R. Reyes Memorial Medical Center approved the study.
AUTHORS CONTRIBUTION
TJCK, RGU, MVB, JMF, data curation, investigation, methodology, software, validation, writing –original draft, writing – review & editing;
TJCK, software, writing – review & editing;
TJCK, RGU, MVB, JMFR, investigation, writing – review & editing; TJCK, RGU, CCL, MVB, JMFR, investigation, writing – review & editing; TJCK, SLC, resources, writing – review & editing;
TJCK, SLC, methodology, writing – review & editing; TJCK, SLC, resources, writing – review & editing; TJCK, writing – original draft;
TJCK, RGU, SLC, writing – review & editing;
TJCK, RGU, SLC, resources, writing – review & editing; TJCK, RGU, SLC, writing – review & editing;
TJCK, JMFR, MJC, conceptualization, data curation, funding acquisition, methodology, supervision, validation, writing – original draft, writing – review & editing
FUNDING
This research received no external funding.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to commercial or financial relationships.
PUBLISHER’S NOTE
This article reflects the views and findings of the authors alone and does not necessarily represent the official position of the author's affiliated organizations, the publisher, editors, or reviewers. We encourage readers to remember that the content presented here does not constitute endorsement or approval by any of the entities above.
Similarly, any products or services mentioned within this article are for informational purposes only. The publisher recommends that readers conduct independent evaluations before making any decisions, as the publisher does not guarantee or endorse any products or services mentioned.
DECLARATION OF USE OF GENERATIVE ARTIFICIAL INTELLIGENCE
No generative AI technologies were used in the writing of this manuscript.
REFERENCES
National Comprehensive Cancer Network. Breast Cancer (Version 5.2021) [Internet]. 2021 [cited 2023 Jul 10]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative Radiotherapy in High-Risk Premenopausal Women with Breast Cancer Who Receive Adjuvant Chemotherapy. N Engl J Med. 1997;337(14):949-55.
Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641-8.
Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional Radiation Therapy in Patients With High-Risk Breast Cancer Receiving Adjuvant Chemotherapy: 20-Year Results of the British Columbia Randomized Trial. J Natl Cancer Inst. 2005;97(2):116-26.
Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomized trial. Lancet Oncol. 2008;9:331-41.
Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomized trial. Lancet. 2008;371:1098-107.
Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513- 20.
Hall E. Radiobiology for the radiologist. 8th ed. Philadelphia: Wolters Kluwer; 2019.
American Society for Radiation Oncology. COVID-19 Recommendations and Information [Internet]. [cited 2023 Jul 10]. Available from: https://www.astro.org/Daily-Practice/COVID- 19-Recommendations-and-Information/Clinical-Guidance
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-49.
Laudico A, Mirasol-Lumague MR, Mapua CA, Uy GB, Toral JA, Medina VM, et al. Philippine Cancer Facts and Estimates [Internet]. Manila: University of the Philippines; 2015 [cited 2023 Jul 10]. Available from: http://www.philcancer.org.ph/wp- content/uploads/2017/07/2015-PCS-Ca-Facts-Estimates_CAN090516.pdf
McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomized trials. Lancet. 2014;383:2127-35.
Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med. 2015;373(19):1877- 80.
Poortmans PM, Weltens C, Fortpied C, Kirkove C, Peignaux-Casasnovas K, Budach V, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I–III breast cancer (EORTC 22922/10925): 15-year randomised, phase 3 trial results. Lancet Oncol. 2020;21(12):1602-10.
Sun GY, Wang SL, Song YW, Jin J, Wang WH, Liu YP, et al. Hypofractionated Radiation Therapy After Mastectomy for the Treatment of High-Risk Breast Cancer: 5-Year Follow-Up Result of a Randomized Trial. Int J Radiat Oncol Biol Phys. 2017;99(2):S3-4.
Ali E, Khalil M. Post-mastectomy Hypofractionation Radiotherapy in Breast Cancer Patients. Cancer Oncol Res. 2014;2(7):87-93.Rastogi K, Jain S, Bhatnagar AR, Bhaskar S, Gupta S, Sharma N. A Comparative Study of Hypofractionated and Conventional Radiotherapy in Postmastectomy Breast Cancer Patients. Asia Pac J Oncol Nurs. 2018;5(1):107-13.
Eldeeb H, Awad I, Elhanafy O. Hypofractionation in post-mastectomy breast cancer patients: seven-year follow-up. Med Oncol. 2012;29(4):2570-6.
Khan AJ, Poppe MM, Goyal S, Kokeny KE, Kearney T, Kirstein L, et al. Hypofractionated Postmastectomy Radiation Therapy Is Safe and Effective: First Results From a Prospective Phase II Trial. J Clin Oncol. 2017;35(18):2037-43.
Koukourakis MI, Panteliadou M, Abatzoglou IM, Sismanidou K, Sivridis E, Giatromanolaki
A. Postmastectomy Hypofractionated and Accelerated Radiation Therapy With (and Without) Subcutaneous Amifostine Cytoprotection. Int J Radiat Oncol Biol Phys. 2013;85(1):e7-13.
Pinitpatcharalert A, Chitapanarux I, Euathrongchit J, Tharavichitkul E, Sukthomya V, Lorvidhaya V. A retrospective study comparing hypofractionated and conventional radiotherapy in postmastectomy breast cancer. J Med Assoc Thai. 2011;94 Suppl 2:S94-102.
Wang S, Fang H, Song Y, Wang W, Hu C, Liu Y, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20(3):352-60.
Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, et al. FAST-Forward Phase III Randomised Controlled Trial of 1-Week Hypofractionated Breast Radiotherapy: 5-Year Results for Efficacy and Late Normal Tissue Effects. SSRN Electronic Journal. 2020. doi:10.2139/ssrn.3564389
Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, version 4.0 [Internet]. [cited 2023 Jul 10]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-6.
Dishe S, Warburton M, Jones D, Lartigau E. The recording of morbidity related to radiotherapy. Radiother Oncol. 1989;16:103-5.
Rubin P, Constine LS, Fajardo LF, Phillips TL, Wasserman TH. RTOG late effects working group. Overview of late effects normal tissues (LENT) Scoring system. Radiother Oncol. 1995;35:9-10.
Pavy JJ, Denekmap J, Letschert J, Littbrand B, Mornex F, Bernier J, et al. EORTC late effects working group. Late effects toxicity scoring: the SOMA scale. Radiother Oncol. 1995;35:11-5.
Denekamp J, Bartelink H, Rubin P. Correction for the use of the SOMA LENT tables. Radiother Oncol. 1996;39:191.
SWOG Cancer Research Network. Statistical Tools [Internet]. 2017 [cited 2023 Jul 10]. Available from: https://stattools.crab.org/
Arsenault J, Parpia S, Goldberg M, Rakovitch E, Reiter H, Doherty M, et al. Acute Toxicity and Quality of Life of Hypofractionated Radiation Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys. 2020;107(5):943-8.
Rudat V, Nour A, Hammoud M, Abou Ghaida S. Better compliance with hypofractionation vs. conventional fractionation in adjuvant breast cancer radiotherapy: Results of a single, institutional, retrospective study. Strahlenther Onkol. 2017;193(5):375-84.
White J, Tai A, Arthur D, Buchholz T, MacDonald S, Marks L, et al. Breast Cancer Atlas for Radiation Therapy Planning: Consensus Definitions [Internet]. 2009 [cited 2023 Jul 10]. Available from: https://www.rtog.org/LinkClick.aspx?fileticket=vzJFhPaBipE%3d&tabid=236
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) [Internet]. 2009 [cited 2023 Jul 10]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06- 14_QuickReference_5x7.pdf
Received: 2024 Jan 22 Received in revised form: 2024 May 28 Accepted: 2024 Jun 11
CITATION
Ngelangel TJC, Uy RG, Ciocon SL, Lo CC, Barcelona MV, Fernandez-Ramos JM, Calaguas MJ. Hypo-fractionated radiotherapy versus conventional radiotherapy in post-mastectomy breast cancer patients: a single institution trohoc study. Philipp J Oncol [Internet]. 2025 [cited 2025 Jun 9];1(1):e005. Available from: [https://www.philsoconc.org/post/hypo-fractionated-radiotherapy-versus-conventional-radiotherapy-inpost-mastectomy]
FIGURES
 |
 |
 |
 |
TABLES
Table 1 Demographic profile of post-mastectomy breast cancer patients who underwent hypofractionated or conventional radiotherapy (N = 87).

Table 2 Histopathologic profile of post-mastectomy breast cancer patients who underwent hypofractionated or conventional radiotherapy (N = 87).

Table 3 Treatment profiles of post-mastectomy breast cancer patients, by type of radiotherapy fractionation (n=87).
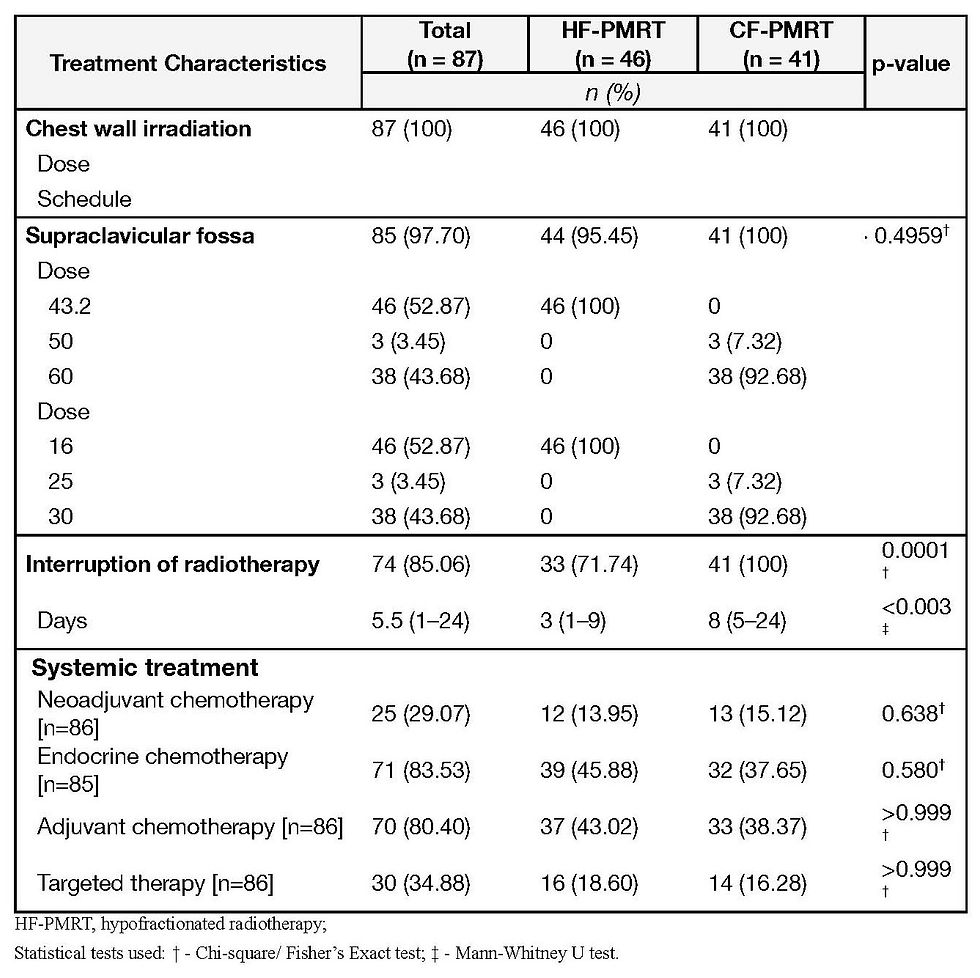
Table 4 Outcomes of post-mastectomy breast cancer patients who underwent radiotherapy (n=87).

Table 5 Adverse Event Outcomes of Post-Mastectomy Breast Cancer Patients Who Underwent Radiotherapy (N = 87).

Comments