Population-Based 5-year Cancer Survival Study (2006-2017) among Filipino Pediatric Cancer Patients
- PJO
- Jun 21, 2025
- 18 min read
Updated: Dec 1, 2025
Population-Based 5-year Cancer Survival Study (2006-2017) among Filipino Pediatric Cancer Patients
Rachael Marie B. Rosario (1-2), Ana Patricia A. Alcasabas (1-3), Corazon A. Ngelangel (1-2), Adriano V. Laudico (1,4), Rica M. Lumague (4), Edmund A. Orlina (4), Cynthia A. Mapua (1,4), Maricar R. Sabeniano (1), Vianna R. Yunque (4), Rauell John Santos (5), Rey Arturo Fernandez (5), Jan Aure Llevado (6), Alyanna Riel Panlilio (6)
1 Philippine Cancer Society Manila Cancer Registry, 2 University of the Philippines-College of Medicine, 3 Philippine Society of Pediatric Oncology, 4 DOH-Rizal Cancer Registry, 5 World Health Organization-WPRO, 6 DOH-Cancer Control Division
Corresponding author: Corazon A. Ngelangel; philippinecancer.org@gmail.com
ABSTRACT
Objective: This retrospective cohort study aims to determine the population-based five-year survival rate of patients aged 0-19 years diagnosed with cancer in the era before the National Integrated Cancer Control Act (NICCA) was signed into law. The study focuses on lymphoid leukemia, Hodgkin's lymphoma, Burkitt's lymphoma, retinoblastoma, nephroblastoma, and osteosarcoma.
Methods: The study utilizes data from two primary sources: the Philippine Cancer Society – Manila Cancer Registry (PCS – MCR) and the Department of Health – Rizal Cancer Registry (DOH-RCR). The cohort includes patients diagnosed between 2006 and 2017, providing a comprehensive dataset for analysis of survival rates in the pre-NICCA period.
Results: The survival rates for the above specific pediatric cancer types in the Philippines were low. Possible barriers include financial constraints, difficulties accessing diagnostic and treatment services, and awareness issues. The Acute Lymphoid Leukemia (ALL) DOH- Childhood Cancer Medicine Access Program (CCMAP) and PhilHealth Z package improved ALL survival rates. The significance of targeted healthcare programs and areas for improvement, such as enhanced access to comprehensive care, is highlighted. The DOH-CCMAP expansion to include other cancers and upgrading children's services in DOH-designated cancer centers across the country, mandated by the National Integrated Cancer Control Act (NICCA), are welcome to control pediatric cancer. In 2030, perhaps cancer survival rates would significantly improve.
Conclusion: The study reveals low survival rates for specific pediatric cancers in the Philippines, highlighting the need for improved access to comprehensive care and targeted healthcare programs. Implementing the National Integrated Cancer Control Act, expanding the DOH- CCMAP, and upgrading children's services in cancer centers nationwide are promising steps toward improving pediatric cancer outcomes in the country.
Keywords: Childhood cancer survival, population-based cancer registry, PCS-MCR, DOH-RCR
INTRODUCTION
The global burden of pediatric cancer is growing, with an estimated 400,000 children developing cancer each year.(1) Disparities in survival rates between high-income countries (HICs) and low-middle-income countries (LMICs) are significant, with average global survival rates of 37%, ranging from 90% in HICs to less than 30% in LMICs.(2-4)
In the Philippines, about 3% of cancers occur in children aged <14 years, and cancer ranks third leading cause of morbidity and mortality.(5) Although developed countries have high survival rates of 80-90%, <30% of children survive in developing countries.(2-3)
Annually, approximately 4700 Filipino children aged 0-19 years are expected to be diagnosed with cancer, and 1700 Filipino childhood deaths will be due to cancer.(6) Unfortunately, although multidisciplinary management is available and could potentially cure 80% of such cases, only about 10-20% attain long-term survival.(5) The obstacles to early detection and effective management of childhood cancer in the Philippines include the lack of prompt recognition of subtle signs and symptoms, patients and parents delaying medical consultations, lack of cancer treatment facilities in the locality, costly treatment, abandonment of treatment, and advanced stages on initial medical consultation.(5) Out of the 20-30% of children diagnosed early, a significant percentage cannot continue follow-up visits or hospitalization.(5) Locally, inequity exists between high-income and low-income families’ capacity to access affordable cancer care.
Stakeholders must implement measures that address the issue of inequity of cancer care in the Philippines by providing support, advocacy, and resources for preventing, detecting, and treating childhood cancer.
In 2018, WHO launched the Global Initiative for Cancer Control (GICC) to address the increasing burden of childhood cancer and reduce survival rate disparities between HICs and LMICs.(6) The GICC aims to raise the global survival rate of children with cancer to at least 60% by 2030, saving an additional one million lives. This goal is supported by the WHO CureAll framework, which outlines programs and priority interventions under four pillars: Centers of excellence and care networks with a sufficient and competent workforce to increase capacity to deliver quality patient-centered services, Universal health coverage by integrating childhood cancer as part of the full range of essential quality-assured services and included in benefit packages, Regimens and roadmaps for diagnosis and treatment that are context appropriate and facilitate delivery of quality services through evidence-based utilization of essential health products, and Evaluation and monitoring with robust information systems.(7-8)
Before the GICC, several cancer control programs had already been implemented in the Philippines. These efforts included the ALL DOH-CCMAP and the PhilHealth Z package, which started in 2012.(9) The DOH-ALL CCMAP provides subsidized drugs to treat children with ALL. The PhilHealth ALL-Z package offers additional support for cancer patients, covering the cost of diagnostic and other treatment services.(9) Before 2022, there was no PhilHealth Z-package nor DOH CCMAP for other childhood cancer types. Some hospitals (e.g., Philippine General Hospital, Philippine Children Medical Center, and National Children Medical Center) have children cancer- focused services with experts in pediatric oncology.
The NICCA was signed into law on 14 February 2019.(10) This Law establishes a comprehensive and integrated approach to cancer control in the Philippines and mandates the development and implementation of a national cancer control program encompassing health promotion, prevention, early detection, diagnosis, treatment, palliative care as well as capacity building, research, and financing mechanisms to improve access to care and support services for cancer patients and their families.
Strengthening local cancer registries towards a national cancer registry measures the country's efforts for pediatric cancer control. Section 28 of NICCA explicitly states the need to establish a national cancer registry, which will be a population-based cancer registry (PBCR) that collects data per geographical region to provide a framework for assessing and controlling the impact of cancer on the community.
Both the GICC and NICCA encourage PBCRs. Unlike hospital-based cancer registry (HBCR), PBCR provides a more comprehensive picture of the cancer burden within a community and enables the monitoring and Evaluation of cancer control activities at the population level. Hospitals are mandated to have medical records (MR), eventually electronic MR, from which they can cull out their own HBCR.
The Philippines has two PBCRs, the Philippine Cancer Society–Manila Cancer Registry (PCS– MCR) and the Department of Health–Rizal Cancer Registry (DOH–RCR), which gather data on cancer, including on children from the Metro Manila 16 cities and 14 municipalities.(11-12) Both registries are recognized by the WHO-International Agency for Research on Cancer (IARC)(13) and produced incidence data published in the International Incidence of Childhood Cancer Volumes 2 and 3. Population-based cancer survival data reflects the impact of national cancer control programs and policies.
We determined the population-based five-year survival rate of pediatric patients diagnosed with select specific cancers from the PCS-MCR and DOH-RCR catchment areas covering the years 2006-2017, serving as a baseline prior to the implementation of NICCA.
METHODS
This project was conducted with the DOH Cancer Control Division and WHO-WPRO. This retrospective cohort included children registered in the PCS-MCR and DOH-RCR, aged 0-19 years, who were diagnosed with primary cancer between 1 January 2006 and 31 December 2017. The children were with histopathological or cytological confirmed diagnosis of cancer. Excluded were those registered based on death certificates or autopsy reports only or with incomplete or missing birth dates, diagnosis, or last known vital status, incoherent data sequences, and non-malignant tumors.
Data extracted from preexisting databases PCS-MCR and DOH-RCR included date of diagnosis, age at diagnosis, sex, cancer type, basis of diagnosis, extent of disease, initial treatment, date of death or date of last contact, vital status, cause of death, and place of death. For cases without death records in the PBCRs, death status was ascertained from the Local Civil Registries.
Descriptive statistics such as mean and standard deviations for quantitative data and frequencies and proportions for categorical data were used to summarize the demographic and clinical characteristics of the study population. Researchers used survival analysis to study the WHO GICC index childhood cancers (lymphoid Leukemia, Hodgkin’s lymphoma, Burkitt’s lymphoma, retinoblastoma, nephroblastoma) and osteosarcoma, a common cancer among adolescent Filipino children. Researchers used Kaplan-Meier survival analysis to estimate the overall 5-year survival rates and median survival times for different groups defined by cancer type, age group, sex, stage, and treatment. Particularly for Leukemia, they compared survival rates between the pre-and post- post-implementation periods of the ALL DOH-CCMAP and PhilHealth Z packages. Log-rank tests were used to assess the differences in survival between these groups statistically.
RESULTS
Data for a total of 2,685 pediatric cancer cases were obtained from the Philippine Cancer Society-Manila Cancer Registry (PCS-MCR) and the Department of Health-Regional Cancer Registries (DOH-RCR). These cases included diagnoses of lymphoid leukemia, Hodgkin's lymphoma, Burkitt's lymphoma, retinoblastoma, nephroblastoma, and osteosarcoma (Table 1). Of these, 2,256 cases (84%) were included in the final analysis (Table 2), representing 58% of all children with cancer recorded during the study period (2006-2017). The combined registries reported a total of 100,567 cancer cases (96,654 adults and 3,913 children) from 2006-2017.
Lymphoid Leukemia
The majority of children diagnosed with lymphoid leukemia (79.4%) received their diagnosis in government hospitals. The most prevalent age groups were 1-4 years (41.41%) and 5-9 years (31.6%), with males constituting 60.2% of the cases (Table 3).
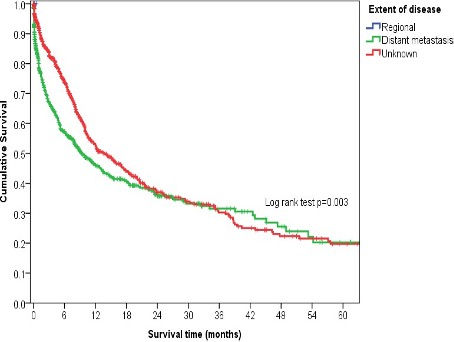
The overall 5-year cumulative survival rate for lymphoid leukemia cases was 19.9%, with a median survival time of 11.6 months (Table 4, Figure 1). Survival outcomes varied by age group: better survival was observed in the 5-9 and 1-4 age groups, while survival was worse for those in the 10-14 and 15-19 years age groups (Figure 2). Although there was a significant difference in survival by extent of disease, the survival times became almost similar as the follow-up period lengthened (Figure 4). Patients who received treatment demonstrated better survival (Figure 5). However, a significant number of patients had unknown disease extent and unknown initial treatment. Two cases were recorded without treatment, while none had localized disease and only two had regional disease.
Furthermore, survival was greater in cases diagnosed after the implementation of the ALL PhilHealth Z packages and DOH-CCMAP (Figure 7).
Hodgkin’s Lymphoma
For children diagnosed with Hodgkin's Lymphoma, the majority (54.6%) were diagnosed in government hospitals. Most patients were aged 15-19 years (67.2%), and just over half were male (57.5%) (Table 5).
The overall 5-year cumulative survival rate for Hodgkin's Lymphoma cases was 24.0%, with a median survival time of 35.1 months (Table 6, Figure 8). Survival was significantly better for patients in the 15-19 and 10-14 age groups, but worse for those aged 5-9 years (Figure 9). Patients who received treatment also showed significantly better survival (Figure 12). Acknowledging data limitations, many patients had an unknown extent of disease and unknown initial treatment; only five cases were recorded as localized.
Burkitt’s Lymphoma
When it came to Burkitt's Lymphoma in children, most (88.2%) were diagnosed in government hospitals. The most common age groups were 1-4 years (40.8%) and 5-9 years (31.6%), with males making up 68.4% of cases. A majority (56.6%) of these cases presented with distant metastasis (Table 7).
The 5-year cumulative survival rate for all Burkitt's Lymphoma cases was 16.7%, with a median survival time of 37.4 months (Table 8, Figure 14). Survival was not significantly different across age group, sex, disease extent, initial treatment, locality, or period of diagnosis (Figures 15-21). This lack of significance may be due to the relatively small number of cases. Similarly, for Burkitt's Lymphoma, many patients had an unknown extent of disease and unknown initial treatment. Furthermore, no cases presented as localized disease, and only one had regional disease.
Retinoblastoma
For children diagnosed with retinoblastoma, most (76.8%) received their diagnosis in government hospitals, and a significant majority (77.2%) were between 1 and 4 years old (Table 9).
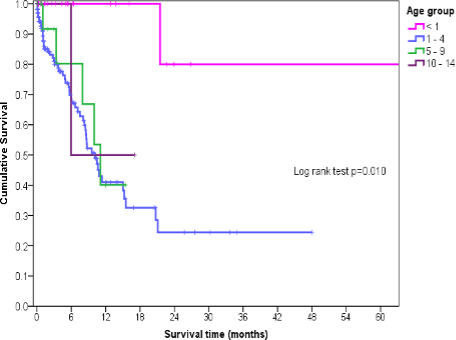
The overall 5-year cumulative survival rate for retinoblastoma cases was 31.6%, with a median survival time of 11.1 months (Table 10, Figure 20). Survival rates varied significantly based on age group (p = 0.010), the extent of the disease (p < 0.001), and the treatment received (p = 0.040) (Figures 21, 23, 24). A considerable number of patients had an unknown extent of disease and unknown initial treatment. While no recorded patients went without treatment, only four cases presented with localized disease.
Nephroblastoma
Most children with nephroblastoma were diagnosed in government hospitals (81.8%) and were aged 1-4 years (61.5%) (Table 11). The overall 5-year cumulative survival rate for nephroblastoma cases was 12.5%, with a median survival time of 7.8 months (Table 12, Figure 26).
Survival outcomes differed significantly by initial treatment (p < 0.001) (Figure 30). This analysis included a substantial number of patients with unknown extent of disease and initial treatment; however, no patients were recorded as receiving no treatment. No significant differences in survival were observed across other demographic or clinical factors analyzed.
Osteosarcoma
Most children with osteosarcoma were diagnosed in government hospitals (76.6%) and predominantly belonged to the 10-14 years (41.1%) and 15-19 years (44.8%) age groups (Table 13). The overall 5-year cumulative survival rate for osteosarcoma was 4.8%, with a median survival time of 11.7 months (Table 14, Figure 32).
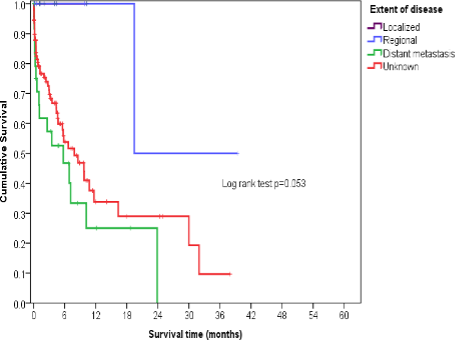
Childhood osteosarcoma survival was significantly higher for localized and regional diseases compared to those with distant metastasis (Figure 35). Additionally, patients who received treatment demonstrated greater survival rates (Figure 36). However, a substantial number of patients lacked complete data regarding the extent of their disease and the initial treatment they received. All recorded patients, however, received some form of treatment.
For all pediatric cancers included in this study, sex was not a significant survival variable. Interestingly, only for osteosarcoma patients did survival appear slightly better in cases recorded by the PCS-MCR compared to those by the DOH-RCR (Figure 37).
DISCUSSION
The first report of population-based cancer survival data in the Philippines came from the IARC's 1998 monograph ‘Cancer Survival in Developing Countries’ (14). This study primarily focused on adult cancers (14-17) and identified a concerning trend: survival rates decreased as the extent of the disease increased for all cancers studied. Improvements in cancer control and making early diagnosis and treatment more accessible remain major challenges.(14-17)
On childhood cancer, Redaniel et al. (18), using the US SEER and the DOH-RCR PCS-MCR 5-year survival data (2001-2005), noted that childhood leukemia and lymphoma relative survival rates were much lower in Filipinos living in the Philippines (32.9 and 47.7%) than in Asian Americans (80.1 and 90.5%) and Caucasians (81.9 and 87%). Achievement of comparable survival rates of Philippine residents lagged by 20 to >30 years compared with patients in the United States. The significant differences in survival estimates of US populations and Philippine residents highlighted the deficiencies of pediatric cancer care delivery in the Philippines. The long survival lag underlines the need for significant improvements in access to diagnostic and treatment facilities. The population-based five-year survival rates for pediatric cancer in the Philippines from 2006 to 2017 revealed critical insights into the challenges and opportunities within the country's healthcare landscape. There were low survival rates for lymphoid Leukemia (19.9%%), Hodgkin’s lymphoma (24.0%), Burkitt’s lymphoma (16.7%), retinoblastoma (31.6%), nephroblastoma (12.5%), and osteosarcoma (4.8%). The analysis revealed survival rates that fall significantly below the WHO GICC target of 60%. This stark contrast highlights the challenges faced by the Philippines in cancer care and control. These findings represent the survival status of Filipino pediatric cancer patients diagnosed before the implementation of the NICCA program.
This study underscores the existing healthcare disparities in Filipino pediatric cancer care. Lack of access to cost-effective and efficient diagnostic and treatment facilities, financial difficulties, and lack of awareness are the major problems faced by Filipino pediatric cancer patients.(5,8,18) The current data analysis shows that most diagnoses of pediatric cancer cases happened in government hospitals. Diagnoses in government hospitals can significantly limit access to care and treatment for childhood cancers, thereby impacting cumulative survival rates. This is especially true for patients with lymphoid Leukemia, Hodgkin's lymphoma, retinoblastoma, nephroblastoma, and osteosarcoma. The top Filipino pediatric cancers are Leukemia (lymphoid). Lymphoma (HL/ NHL), CNS neoplasms (astrocytoma), bone tumors (osteosarcoma), soft tissue sarcoma (rhabdomyosarcoma), retinoblastoma, germ cell tumor (CNS), hepatic tumor (hepatoblastoma), and nephroblastoma, in descending order of incidence (PCS-MCR, 2013-2017).(19)
The quality of care pediatric cancer patients receive significantly impacts their outcomes. Tertiary hospitals with pediatric oncologists, pediatric oncology units, and access to diagnostic and treatment modalities like MRI, PET-CT scan, immunophenotyping, and cytogenetics can provide more comprehensive care that may improve the survival rates of pediatric cancer patients. Osteosarcoma, for example, was noted to have a survival difference between registries, with slightly better survival for those in PCS-MCR. PCS-MCR covers four significant cities: Manila City, Quezon City, Pasay City, and Caloocan City. Critical government hospitals located in these areas include the Philippine General Hospital, National Children's Hospital, and Philippine Children's Medical Center, which cater to pediatric oncology cases and are end-referral hospitals; private tertiary cancer centers are also here like Manila Doctor’s Hospital, Santo Tomas Hospital and Medical Center, St Luke’s Medical Center, Chinese General Hospital, and Manila Medical Center. DOH-RCR covers Las Pinas City, Makati City, Malabon City, Mandaluyong City, Marikina City, Muntinlupa City, Navotas City, Paranaque City, Angono, Antipolo, Baras, Binangonan, Cainta, Cardona, Jala-Jala, and Montalban/Rodriguez - a larger area, but with less government and private specialty hospitals.
Implementing the DOH ALL-CCMAP and PhilHealth ALL Z packages in 2012, which provided diagnostics and drugs for patients diagnosed with acute lymphocytic/lymphoblastic Leukemia, improved the survival rates of children with this disease. This finding suggests that including such programs can positively impact pediatric cancer survival in the Philippines. Since 2022, the DOH-CCMAP and the Cancer Assistance Fund mandated by the NICCA program have provided subsidized drugs and diagnostics for other childhood cancers.
Differences in survival between age groups for lymphoid Leukemia, Hodgkin’s lymphoma, osteosarcoma, and retinoblastoma indicate the need for public campaigns to increase awareness of pediatric cancer signs and symptoms. The challenge now is reaching out to all these children for early diagnosis and prompt, complete treatment from a nationwide chain of pediatric cancer clinics.
This study found that the PBCR data for all cancer types included many cases with missing information on the disease's extent and the initial treatment received. Hospital-based cancer registries (HBCRs) typically capture more detailed information on the extent of cancer disease and initial treatment than population-based cancer registries (PBCRs). This is crucial because the extent of the disease and the initial treatment a patient receives significantly impact their clinical outcome. It would be best if the hospitals wherein the PBCR data were collected would have themselves PBCR to feed into the PBCR for completeness of cancer data.
An initial significant limitation in the cancer survival analysis is the lack of access to the country’s death registry (Philippine Statistics Authority (PSA)), governed by the country's Data Privacy Act. Deaths of registry cases not occurring/ recorded in the PBCR catchment area must be validated via the PSA. The Philippine Statistics Authority (PSA) already has an official process for requesting data through a formal Data Sharing Agreement. Existing collaborations with PhilHealth, the Philippine National Police, and other government agencies exemplify this process, where data is shared at no cost. A supporting mandate of the requesting agency is a prerequisite for the collaboration. NICCA Section 28 mandates the establishment of a National Cancer Registry and Monitoring System, which includes the PBCR.
Researchers underscore the significance of conducting regular PBCR survival studies.(20) This highlights the importance of seamlessly integrating this data into assessing and planning the country's cancer control efforts. It is imperative to grant PBCRs access to the national death registry, necessitating collaborative efforts to streamline regulatory processes and ensure compliance with data privacy regulations. It is recommended that we focus on ongoing capacity building for PBCRs, including training initiatives and resource provisioning, to optimize the efficiency of data collection.
Continuing cancer registry, both hospital-based and population-based, and continuing operations of pediatric cancer clinics across the country with the support of the DOH-designated CCMAP sites and Cancer Centers, all under the mandate of NICCA, would again look for the impact of these programs via a survival analysis in 2030 at the earliest.
Further, public awareness campaigns focusing on pediatric cancers should be initiated to promote early intervention and treatment-seeking behaviors. Significantly, these recommendations extend beyond research, emphasizing the importance of integrating findings into cancer control policies. Policymakers are urged to consider the study's implications in shaping evidence-based cancer control strategies, with a focus on improving diagnostic and treatment accessibility, fostering financial support mechanisms, and developing targeted public health campaigns.
CONCLUSION
By prioritizing accessibility, awareness, and targeted healthcare interventions, the Philippines can significantly improve the landscape of pediatric cancer care, offering better outcomes for the younger population affected by this formidable disease.
A multidimensional approach involving pediatric cancer advocates, communities, healthcare authorities, regulatory bodies, research institutions, and policymakers, fortified by quality cancer survival studies, is essential to driving positive advancements in pediatric oncology care and cancer control policies in the Philippines.
DATA AVAILABILITY STATEMENTS
Data is available at the Philippine Cancer Society Office.
ETHICS STATEMENT
Ethics approval was given by SJREB of DOH.
AUTHORS CONTRIBUTION
All authors contributed to the writing of the manuscript as well as the collection of data.
FUNDING
WHO
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to commercial or financial relationships.
PUBLISHER’S NOTE
This article reflects the views and findings of the authors alone and does not necessarily represent the official position of the authors' affiliated organizations, the publisher, editors, or reviewers. We encourage readers to remember that the content presented here does not constitute endorsement or approval by any of the entities above.
Similarly, any products or services mentioned within this article are for informational purposes only. The publisher recommends that readers conduct independent evaluations before making any decisions, as the publisher does not guarantee or endorse any products or services mentioned.
DECLARATION OF USE OF GENERATIVE ARTIFICIAL INTELLIGENCE
No generative AI technologies were used in the writing of this manuscript.
REFERENCES
Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol. 2019;20(4):483-493.
Rodriguez-Galindo C, Friedrich P, Morrissey L. Global challenges in pediatric oncology. Curr Opin Pediatr. 2015;27(1):3-14.
Atun R, Bhakta N, Denburg A, Frazier AL, Friedrich P, Gupta S, et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol. 2020;21(4):e185-e224.
Koczwara B, Chan A, Jefford M, et al. Cancer Survivorship in the Indo-Pacific: Priorities for Progress. JCO Glob Oncol. 2023;(9).
Lecciones JA. The Global Improvement of Childhood Cancer Care in the Philippines. Cancer Control. 2015;22(2):217-223.
World Health Organization Western Pacific Region. Cancer control legislation in the Philippines a step in the right direction to improve childhood cancer management [Internet]. 2021 [cited 2023 Jul 10]. Available from: https://www.who.int/philippines/news/feature- stories/detail/cancer-control-legislation-in-the-philippines-improve-childhood-cancer- management
World Health Organization. WHO Global Initiative for Childhood Cancer [Internet]. 2021 [cited 2023 Jul 10]. Available from: https://www.who.int/initiatives/who-global-initiative-for- childhood-cancer
Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology as a Global Challenge. J Clin Oncol. 2015;33(27):3065-3073.
Philippine Health Insurance Corporation. PhilHealth Circular No. 2017 – 0016: Enhanced Package Rate for the Z Benefits for Standard Risk Acute Lymphocytic (Lymphoblastic) Leukemia. 2017.
Philippine National Integrated Cancer Control Act, Republic Act No. 11215 (2019).
Philippine Cancer Society. PCS Manila Cancer Registry [Internet]. [cited 2023 Jul 10]. Available from: https://www.philcancer.org.ph/index.php/educational/cancer-registry
Caampued MS. Comprehensive Assessment of Cancer Registries in the Philippines. WHO- Philippines and Alliance for Improving Health Outcomes; 2021.
Parkin DM. Mission Report on Cancer Registration in the Philippines [Internet]. Department of Health; 2011 [cited 2023 Jul 10]. Available from: https://doh.gov.ph/sites/default/files/health_programs/Mission%20Report%20on%20Cancer%20Registration%20in%20the%20Philippines.pdf
Sankaranarayanan R, Black R, Parkin D. Cancer survival in developing countries, 1998. Lyon: International Agency for Research on Cancer; 1998.
Laudico A, Mapua C. Cancer survival in Manila, Philippines, 1994-1995. IARC Sci Publ. 2011;162:147-50.
Laudico AV, Mirasol-Lumague MR, Mapua CA, et al. Cancer Incidence and Survival in Metro Manila and Rizal Province, Philippines. Jpn J Clin Oncol. 2010;40(7):603-612.
Redaniel MT, Laudico AV, Mirasol-Lumague MR, et al. Cancer survival differences between European countries and an urban population from the Philippines. Eur J Public Health. 2010;20(5):560-565.
Redaniel MT, Laudico A, Mirasol-Lumague MR, et al. Geographic and ethnic differences in childhood leukaemia and lymphoma survival: comparisons of Philippine residents, Asian Americans and Caucasians in the United States. Br J Cancer. 2010;103(1):149-154.
Philippine Cancer Society - Manila Cancer Registry, 2013-2017 (0 – 19 years old) [Internet]. 2017 [cited 2024 Jan 3]. Available from: https://drive.google.com/file/d/1LIvus8tsYvcGq6oi4vvJ3WaqP9wgl76n/view
Allemani C, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075.
Received: 2024 Jan 22 Received in revised form: 2024 May 28 Accepted: 2024 Jun 11
CITATION
Rosario RMB, Alcasabas APA, Ngelangel CA, Laudico AV, Lumague RM, Orlina EA, Mapua CA, Sabeniano MR, Yunque VR, Santos RJ, Fernandez RA, Llevado JA, Panlilio AR. Population-based 5-year cancer survival study (2006-2017) among Filipino pediatric cancer patients. Philipp J Oncol [Internet]. 2025 [cited 2025 Jun 9];1(1):e004. Available from: [https://www.philsoconc.org/post/population-based-5-year-cancer-survival-study-2006-2017-among-filipino-pediatric-cancer-patients]
FIGURES
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
TABLES
Table 1 Number and proportion of microscopically verified and death certificate-only cases by childhood cancers, RCR and MCR, 2006-2017.

Table 2 Number and proportion of included and excluded cases by childhood cancers, RCR and CR, 2006-2017.

Table 3 Characteristics of childhood lymphoid leukemia, RCR and MCR, 2006-2017.

Table 4 Childhood lymphoid leukemia survival, RCR, and MCR, 2006-2017.

Table 5 Characteristics of childhood Hodgkin lymphoma, RCR and MCR, 2006-2017.

Table 6 Childhood Hodgkin lymphoma survival, RCR and MCR, 2006-2017.
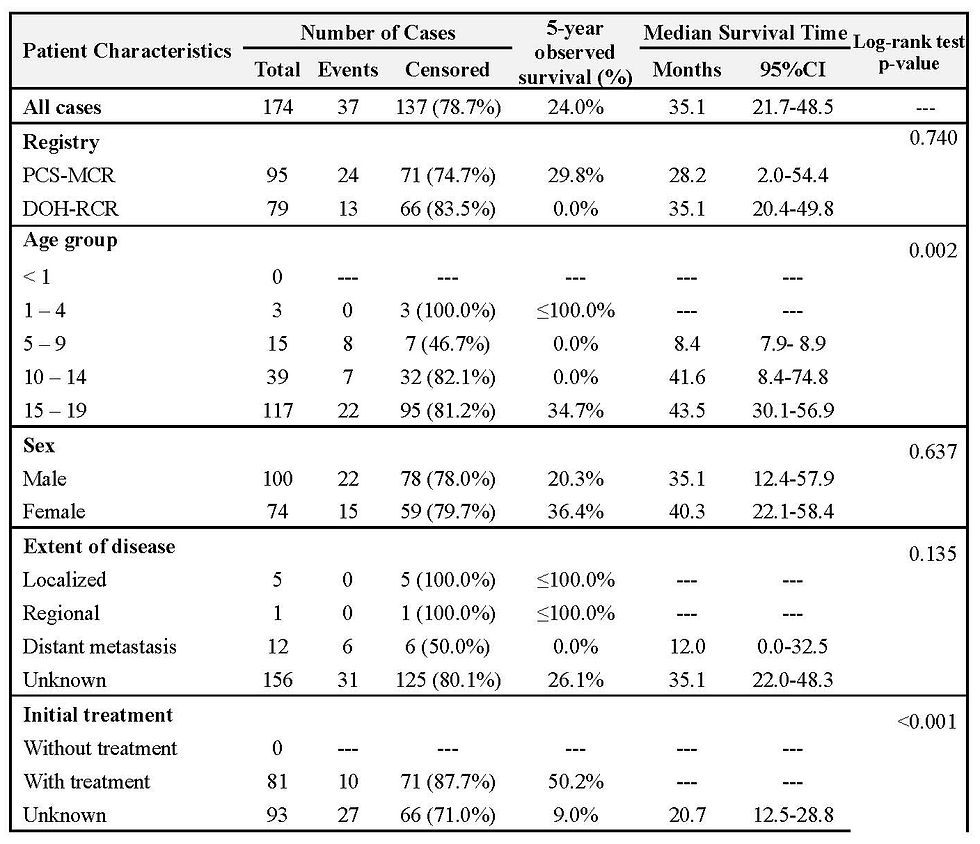
Table 7 Characteristics of childhood Burkitt’s lymphoma, RCR, and MCR, 2006-2017.

Table 8 Childhood Burkitt’s lymphoma survival, RCR and MCR, 2006-2017.

Table 9 Characteristics of childhood retinoblastoma, RCR and MCR, 2006-2017.

Table 10 Childhood retinoblastoma survival, RCR and MCR, 2006-2017.

Table 11 Characteristics of childhood nephroblastoma, RCR and MCR, 2006-2017.

Table 12 Childhood nephroblastoma survival, RCR and MCR, 2006-2017.

Table 13 Characteristics of childhood osteosarcoma, RCR and MCR, 2006-2017.

Table 14 Childhood osteosarcoma survival, RCR and MCR, 2006-2017.


Comments